Welcome to the Prothena ASCENT clinical trials website!
About the ASCENT Clinical Trials
The ASCENT clinical trials are evaluating an investigational drug called PRX012.
Inclusion/Exclusion Criteria
Clinical Trial Locations
Selected Key Eligibility Criteria

ASCENT-1 Clinical Trial
Key Inclusion Criteria
- Adults 60 to 85 years of age
- Diagnostic criteria of AD based on one of the following:
- Probable AD with evidence of the AD pathophysiological process
- Mild cognitive impairment due to AD
- Gradual and progressive change in memory function for ≥6 months

ASCENT-2 Clinical Trial
Key Inclusion Criteria
- Adults 55 to 85 years of age
- Diagnostic criteria of AD based on one of the following:
- Probable AD with evidence of the AD pathophysiological process
- Mild cognitive impairment due to AD
- Gradual and progressive change in memory function for ≥6 months
These are not complete lists of eligibility criteria.
Please contact a clinical trial representative for more information.
About the ASCENT Clinical Trials

ASCENT-1 Clinical Trial
Primary Objective
To evaluate the safety, tolerability, and immunogenicity of PRX012 when administered as a single dose in patients with mild AD or mild cognitive impairment due to AD.
Clinical Trial Design
This clinical trial will be conducted in multiple cohorts in patients with biologically confirmed AD to assess safety, tolerability, and immunogenicity at escalating dose levels and to characterize the safety and pharmacokinetics (PK) profile of PRX012.
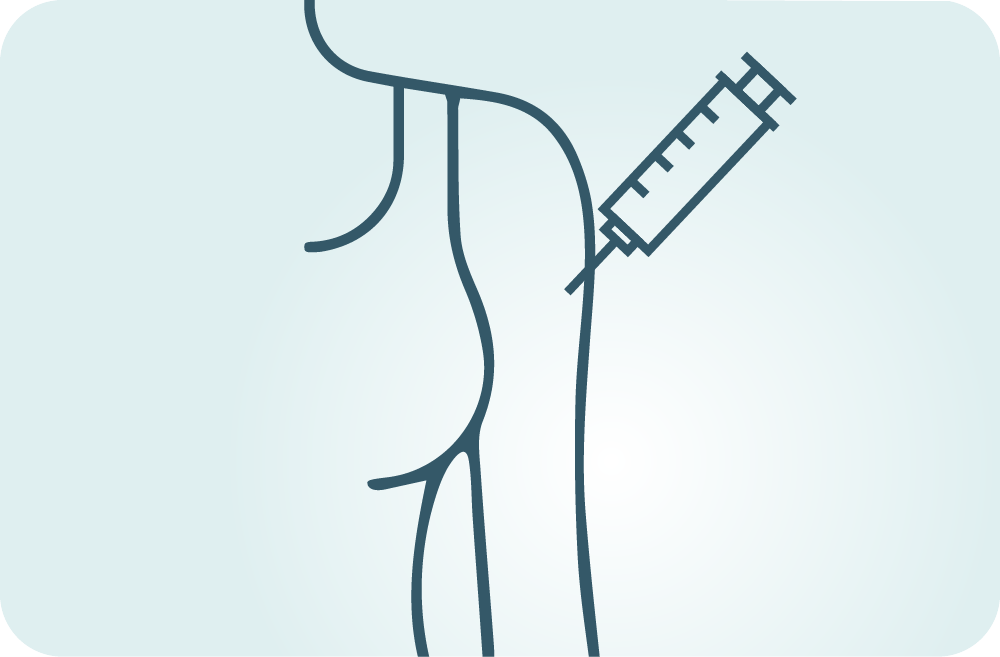
The study drug consists of PRX012 or placebo administered subcutaneously.

ASCENT-2 Clinical Trial
Phase 1, placebo-controlled, multiple ascending dose clinical trial of PRX012 in adults aged 55 to 85 with probable AD or mild cognitive impairment due to AD.
Primary Objective
To evaluate the safety, tolerability, and immunogenicity of PRX012 after multiple subcutaneous doses.
Clinical Trial Design
Eligible participants are randomized to receive either placebo or PRX012 at predetermined dose strengths. Participants will be monitored for safety and tolerability throughout the clinical trial, including a follow-up visit after the final dose. Pharmacokinetics (PK) and pharmacodynamics (PD) will also be measured in all participants during the treatment period of the clinical trial.
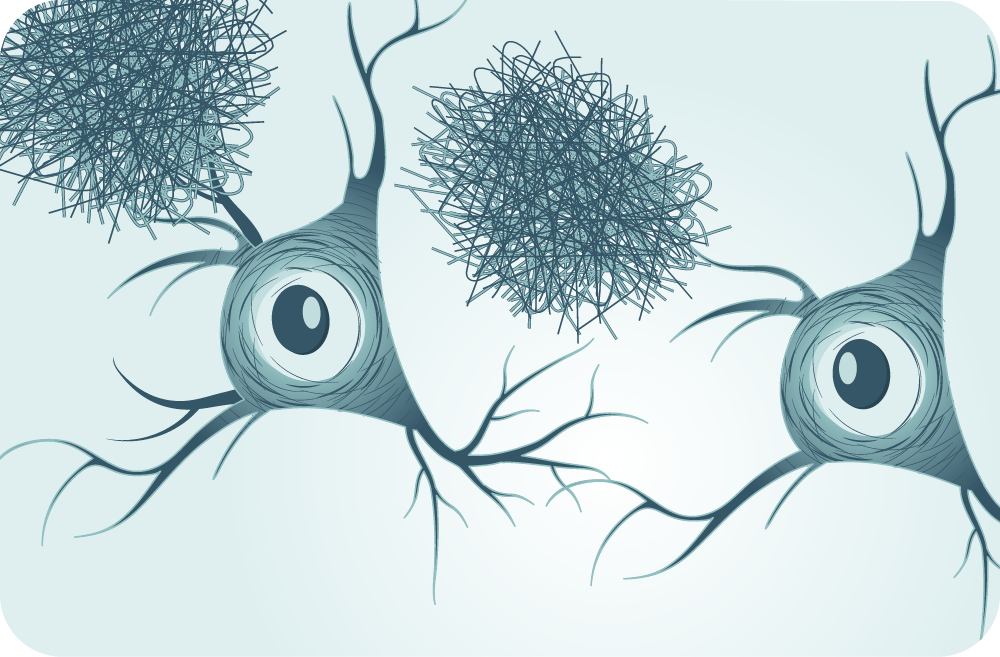
About the Investigational Drug PRX012
PRX012 is a humanized IgG1 monoclonal antibody designed to target and clear aggregated toxic forms of amyloid beta, including soluble oligomers and protofibrils and insoluble plaque.
How is the study drug given?
What to Expect During the ASCENT Clinical Trials
Clinical Trial Duration

ASCENT-1 Clinical Trial
Participants will take part in the ASCENT-1 Clinical Trial for approximately 6 months and have in-person visits, including a short overnight stay at the research clinic, for safety and PK assessments.

ASCENT-2 Clinical Trial
Participants will take part in the ASCENT-2 clinical trial for approximately 1 year and have in-person visits for safety, PK and PD assessments.
How do I refer my patients?
Or you may contact the trial team at (877) 618-3004.
Common Questions
Will compensation be provided to participants?
Compensation will be provided for clinical trial participation, depending on the study site location. Clinical trial related travel reimbursements may be provided. No health insurance is needed.
What is the length of the ASCENT clinical trials?
ASCENT-1 Clinical Trial: Participants will be enrolled for approximately 6 months.
ASCENT-2 Clinical Trial: Participants will be enrolled for approximately 1 year.
WHERE WE ARE
SITE LOCATIONS
Help spread awareness of the ASCENT clinical trials by sharing with others!
To share this clinical trial information via email, click here.


 English - US
English - US